Supply Chain Management | Governance and Integrity | Taiwan Blood Services Foundation ESG
Supply Chain Management
We are committed to establishing a positive cycle supply chain. We have formulated high standards for quality, delivery, price, and process technology, as well as ensuring the quality of the supply chain from three directions: risk control, supply optimization, and local supply.
Supplier (vendor) management
Procurement (medical supply) management
In order to ensure the sufficient supply for materials for blood donation operations and the quality, we have set up a dedicated unit to be responsible for the procurement and management of medical equipment. Procurement review meetings are held on a regular basis to discuss procurement-related issues or cases, and electronic operations have been adopted to significantly enhance the efficiency of procurement operations and reduce the amount of paper used. At the same time, priority is given to local suppliers in Taiwan for procurement and contracting. In 2023, there were 1,668 suppliers, and the total procurement amount was NTD 2,786,638,541. We vow to create local employment opportunities, and continue to target the selection of green- and safety-certified products, in order to fulfill our responsibility for sustainability.
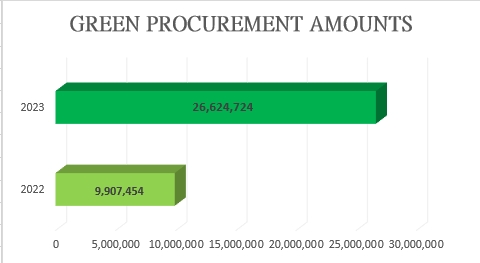
To deepen the concept of sustainable environmental protection, implement green procurement, and enhance efficiency management, our organization has introduced an information-based financial management system since 2023. From the origin of purchase requests to procurement and payment, each stage of operation includes friendly reminders to internalize the concept of sustainability among our colleagues. As a result, the amount of green procurement in 2023 grew 2.69 times compared to 2022.
In order to ensure the quality of the purchased goods delivered by the vendors, we have set up the acceptance criteria. For the management of medical materials, we also have set up the warehouse management system and warehouse management regulations to perform quality control, and adopted the "Blood Management Information System" (BMS) to fill in the online report to strictly control the defective products of the medical devices. The procedure for the notification of the serious adverse events of the medical materials has also been set up.
Medical Supplies Adverse Event Reporting System
Blood Management Information System
Guidelines for handling non-conforming items in place. A non-conforming item found must be reported internally and reported to the management review meeting.
Medical Supplies Adverse Event Reporting
Reporting procedures for serious adverse events of medical materials in place. When defective products are found, they are handled and evaluated according to the defective medical material reported.
Medical Supplies Adverse Event Reporting System of the Ministry of Health and Welfare
According to the Regulations for Reporting Serious Adverse Events of Medical Devices. The scope of reporting of medical supplies is based on the Medical Supplies Adverse Event Reporting System of the Ministry of Health and Welfare.




